Prescription drugs have become a major part of preventative care in our nation and many people rely daily on pharmaceuticals to treat a variety of health issues. Mass production is needed to meet the growing needs of this industry and manufacturers rely on color consistency for quality and safety. This technology can be used to verify batch concentrations, leading to rapid and effective analysis for a variety of pharmaceutical products.
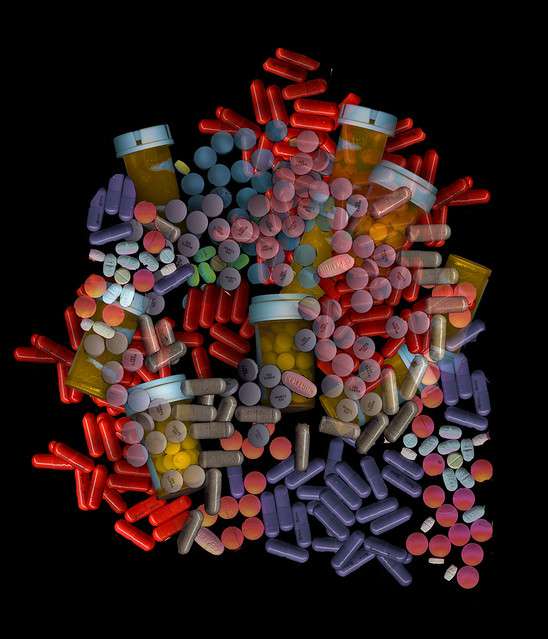
Color consistency can be used to verify batch concentrations in a variety of pharmaceutical products and prescription medications.
Image Source: Flickr user psyberartist