Color surrounds us every moment of our lives and affects our emotions, behaviors and beliefs in large and small, conscious and unconscious ways. Color can set a mood, warn us of danger, give us critical information and even bring us joy. Despite the universal presence of color, describing it remains elusive, in part due to variations in color perception from person to person and in part due to a lack of descriptors for each of the millions of shades seen by the human eye.
Instrumental color measurement moves beyond the limits of human perception and vocabulary and allows us to capture color information as objective data, creating a common language of color that is essential for communication within and between industries around the world, ranging from food and beverage to pharmaceuticals. The two most advanced color measurement instrument types are colorimeters and spectrophotometers, both of which use sophisticated technologies to accurately and precisely quantify and define color.
While closely related, these instruments have unique qualities that may make one more suitable than the other for a particular type of measurement. Understanding the characteristics of a colorimeter vs. spectrophotometer can help you select the best tool for your application.
What Is a Colorimeter?
A colorimeter is designed to perform a type of psychophysical sample color analysis, which means its measurements correlate to human color perception. In other words, it is designed to see color the way we do.
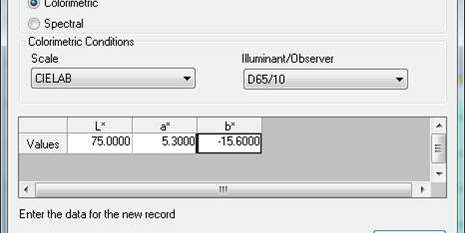
Its results are direct and read as tristimulus values. A tristimulus value is one that identifies a color with characters that represent different dimensions of its visual appearance. A tristimulus value may contain values like X, Y and Z or L, a and b. The “gold standard” for tristimulus colors is the CIE Color System, developed by the International Commission on Illumination — the CIE in the title stands for the French version of their name.
There are a few unique components involved in a colorimeter.
- Illuminant: The illuminant represents a specific light source, such as daylight or incandescent light, to project consistent brightness onto the object. In a colorimeter, an illuminant is fixed.
- Observer: The standard observer offers a specific field of view with which to analyze the colors. A colorimeter usually uses a 2-Degree Standard Observer, which is suitable for color evaluation and quality control.
- Tristimulus absorption filter: The absorption filter isolates specific wavelengths to be applied to the sample.
Types of Colorimeters
Colorimeters are essential in determining color objectively and accurately. Their different varieties measure color to varying depths and degrees. Types include:
- Densitometers: These measure the darkness level, or density, of semi-transparent material.
- Photometers: Color photometers measure how color is transmitted and reflected.
How Does a Colorimeter Work?
A colorimeter’s usage is often based on the Beer-Lambert law, which tells us that the concentration of a solute is proportional to its absorbance. The colorimeter starts with a simple light source. With the help of a lens and tristimulus absorption filters, the beam of light becomes a single, focused wavelength which then moves through to the sample solution. On the other side of the solution is a photocell detector that identifies how much of the wavelength got absorbed. The detector is connected to a processor and digital display that offers a readable output of the results.
Now that you know how it works, let’s take a look at the pros and cons of a colorimeter.